The University of Alabama School of Nursing has recently published their findings from a study by Dr. Michael Callihan, PhD, RN, CEN, NRP and his team who investigated the usability of the SlateSafety wearable device for physiological monitoring. During the study physiological data was collected on a team of nurses performing job-specific tasks in a moderate or hot environment while wearing PPE. While performing tasks the study participants wore the SlateSafety(SS) device, a Polar 10 monitor, and had taken an e-Celsius ingestible pill. Data from each device was compared for correlation and accuracy.
The study entitled: Comparison of SlateSafety Wearable Device to Ingestible Pill and Wearable Heart Rate Monitor was published in a special topic issue of technologies highlighting personal health and well-being through intelligent systems and wearable technologies.
In the study, the SlateSafety wearable compared favorably to the ingestible pill and heart rate monitor with a number of important findings presented including:
- The mean difference in temperature between the ingestible pill and the SlateSafety device was −0.03 (meaning the SS temperature is on average 0.03 C higher) with a standard deviation of 0.3 (95% confidence interval −0.62, 0.57).
- A Root Mean Square(RMSE) of 0.27C and Mean Absolute Error(MAE) of 0.25C between temperature collection from the ingestible pill and the SlateSafety device.
- Qualitatively the SlateSafety device received high marks on comfort and range of motion with all participants reporting that they would prefer the wearable device to the ingestible pill for core temperature monitoring.
Study and Findings
During the study, nursing participants were asked to perform cognitive tasks on a computer and then proceed to a simulated hospital room. The simulation room was temperature controlled to either 71 °F (moderate condition) or 85 °F (hot condition) and at a humidity of approximately 40%, depending upon the condition assigned to the group. The study participants entered the room with a partner and were then asked to perform CPR on a high-fidelity simulator rotating every two minutes for a total of fifteen minutes.
Physiological data were collected from the participants using the SlateSafety wearable device, which collected both heart rate and temperature data, the e-Celsius ingestible pill, which collected temperature data, and the Polar 10 monitor, which collected heart rate data.
Qualitative Findings
In addition to the physiological data being recorded, participants were also asked about the comfort of the wearable devices and if there were any restrictions on the range of motion while completing the simulation tasks. They were then asked to rate the device comfort on a scale of 1–10(1 being the lowest and 10 being the highest comfort level)and the impairment of motion.
Participants reported high comfort and high range of motion while wearing the SS system, with all participants reporting the range of motion for the SS as demonstrating no impairment from normal. All participants reported that they would prefer the wearable device to the ingestible pill for core temperature monitoring. Additionally, in comparison to the other two systems, the continuous monitoring ability of the SS system performed well. It was remarked that the SlateSafety device was comfortable to wear and easily monitored multiple participants from a distance.
Quantitative Findings
During the study, the collected data of interest included heart rate and temperature data as collected using the SS wearable device compared to the e-Celsius ingestible pill and the Polar 10 heart rate monitor. Data was collected within the SS system every minute, the e-Celsius reported data at 30-s intervals, and the Polar 10 system reported data every second. All data was normalized to the minute for comparison.
Heart Rate
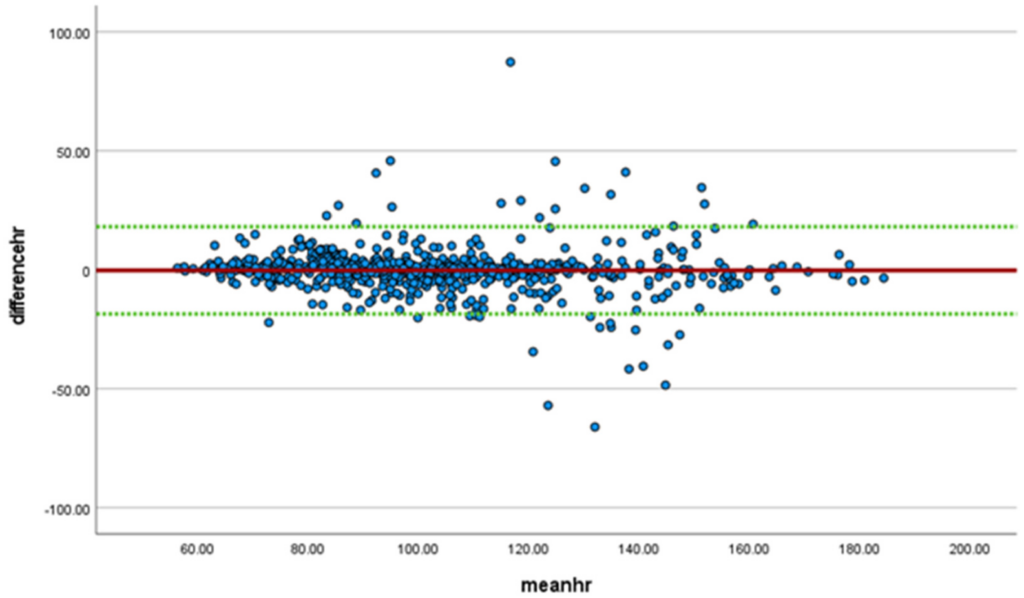
Heart rate was monitored in all participants comparing data from the SS wearable to the Polar 10 device with differences and means plotted in a Bland-Altman plot (Figure 2).Linear regression demonstrated a significant bias (t = −2.6, p = 0.009), with the heart rate being higher for the SS than the Polar system, with a mean difference of −0.23 (meaning SS heart rate is on average 0.23 beats higher) and a standard deviation of 9.4 (95% confidence interval −18.6, 18.1).
From the study:
A significant correlation of 0.926 (p < 0.001) with a 95% confidence interval of 0.915–0.935 was noted between the SS and Polar 10 systems during the collection period, with an intraclass correlation coefficient (ICC) of 0.961 (p < 0.001, 95% CI 0.955–0.966). Aggregate heart rate data demonstrated a Root Mean Square Error (RMSE) of 9.74, a Mean Absolute Error (MAE) of 5.10, and a Mean Bias Error of 0.35 beats per minute higher. Accuracy, as determined by the percentage in the difference between the expected (Polar 10) and the collected (SS), ranged from −16.7% to a 1.84% difference for the peak heart rate and −1.73% to 5.06% for the average heart rate. The accuracy for the average of all heart rates was −1.65% and 1.08% for the peak and average heart rates, respectively.
Core Temperature
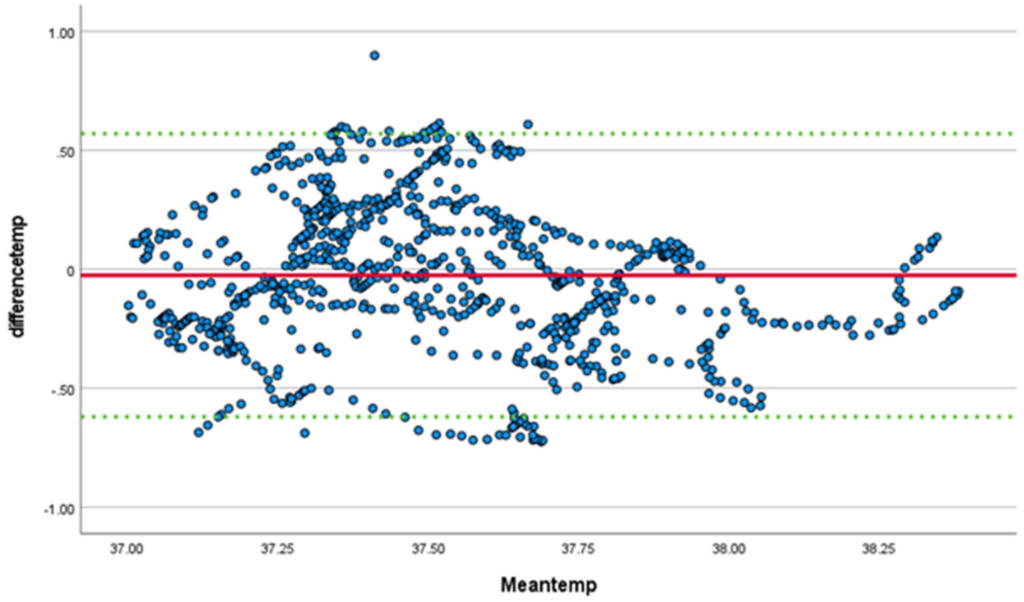
Core temperature monitoring was completed for all participants using the SS wearable device and the e-Celsius ingestible pill, with differences and means plotted in a Bland-Altman plot(Figure 3). Linear regression demonstrated a significant bias (t = −4.6, p < 0.001) toward the SS device, which reported a higher temperature. The mean difference in temperature between the ingestible pill and the SS device was −0.03 (meaning the SS temperature is on average 0.03 C higher) with a standard deviation of 0.3 (95% confidence interval −0.62, 0.57)
From the Study:
Aggregated data demonstrated a mean error bias of 0.04 C higher for the SS system with an RMSE of 0.27 C and an MAE of 0.25 C. A significant correlation (0.595, p < 0.001, 95% CI 0.550–0.635; ICC 0.742, p < 0.001, CI 0.705–0.774) was noted between the SS wearable and the ingestible pill. Accuracy as determined by the percentage difference between the expected (e-Celsius) and the collected (SS) ranged from −1.87% to 1.29% for the peak and −1.45% to 1.98% for the average. The overall accuracy for the averages of the whole were −0.13% and 0.09% for the peak and average core temperatures, respectively.
Conclusion
The study demonstrated that the SlateSafety wearable device offers a reliable and accurate means of monitoring the core temperature and heart rate in a comfortable manner. Its accuracy remained consistent in both hot and moderate simulated environments and was not hindered by participant use of impermeable protective gear. While the objective of the study was not to demonstrate an objective winner, it was noted that during this study the other monitoring systems recorded limitations during real-time monitoring at moments when the participants were facing the greatest danger of heat exhaustion.
Read the full study here.

